Fizz or Fail: Unpacking the Power of Vinegar and Baking Soda
By Brian on September 8, 2025

The Truth About This Popular Cleaning Combination
Vinegar and baking soda have become the go-to cleaning duo for homeowners seeking natural, non-toxic alternatives to harsh chemicals. You’ve probably seen the dramatic fizzing reaction and wondered if it’s as powerful as it looks.
Quick Answer: What You Need to Know About Vinegar and Baking Soda
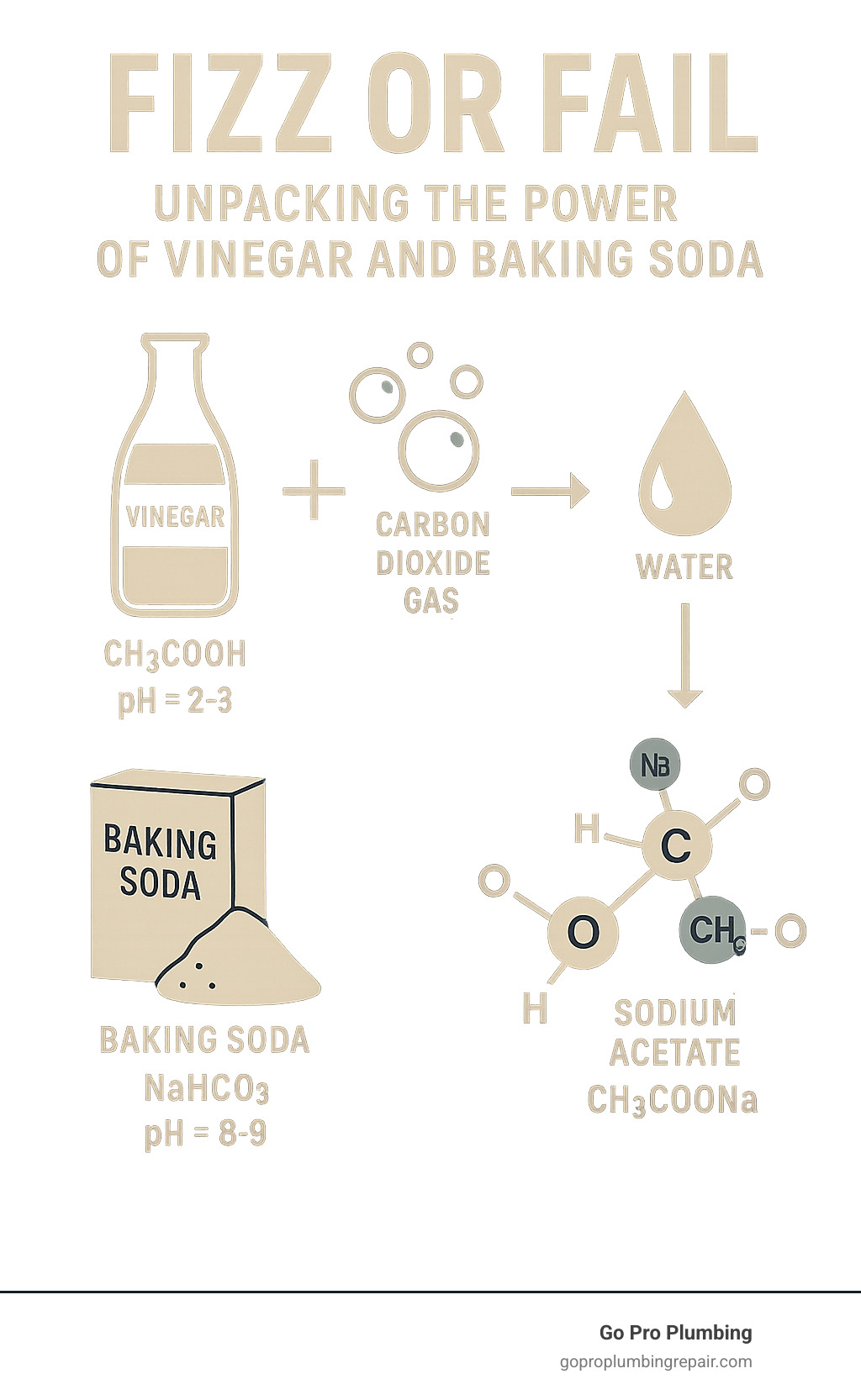
- The Reaction: Acid (vinegar) + Base (baking soda) = Carbon dioxide gas + Water + Salt
- The Reality: They neutralize each other, creating mostly salty water
- Best Use: Often more effective when used separately for different cleaning tasks
- Good For: Drain clogs (mechanical action), deodorizing, some stain removal
- Not Good For: Disinfecting, marble surfaces, sealed containers
The truth is more nuanced than the internet would have you believe. While this pantry staple combination can work for specific tasks, the impressive fizzing doesn’t always translate to superior cleaning power.
As one Reddit user put it: “You’ll be cleaning with ‘water’, essentially” once that satisfying bubble reaction ends. But don’t write off this duo entirely – understanding when and how to use them makes all the difference.
Whether you’re dealing with a stubborn drain clog or just want to reduce chemicals in your home, let’s separate the science from the hype.
Vinegar and baking soda word roundup:
The Science of the Fizz: What Happens When You Mix Vinegar and Baking Soda?
The fizzy reaction when you mix vinegar and baking soda is a classic science experiment, but understanding the chemistry is key to using them effectively for cleaning. It’s a simple acid-base reaction. Vinegar is a mild acetic acid (pH 2-3), while baking soda (sodium bicarbonate) is a base (pH 9).
When combined, they react to form carbonic acid, which is unstable and immediately decomposes into water and carbon dioxide gas. This rapid release of gas creates the signature fizzing and bubbling. In fact, one tablespoon of baking soda can produce over five liters of carbon dioxide when mixed with enough acid, a powerful reaction for a simple household mixture.
The Neutralization Myth: Are You Just Making Salty Water?
Once the fizzing stops, the cleaning power diminishes significantly. The acid and base neutralize each other, leaving you with water, carbon dioxide (which escapes), and sodium acetate—a type of salt with very weak cleaning properties. As chemists like Bill Wuest, PhD confirm, you’ve essentially turned two effective cleaners into salt water. The impressive bubbling can create a placebo effect, making it seem more powerful than it is.
The Power of the Bubbles: Mechanical Cleaning Explained
However, the fizz itself isn’t useless. The physical agitation from the escaping gas bubbles provides a ‘mechanical cleaning’ effect. Think of it as a tiny, scrubbing action that can loosen debris in hard-to-reach places like drains or bottle necks. This action is great for lifting light dirt and food particles, especially in confined spaces where you can’t scrub manually. While it won’t disinfect or remove tough stains, this mechanical force is the primary benefit of combining the two ingredients.
The Power of One: When to Use Them Separately for Best Results

While the fizz is satisfying, vinegar and baking soda are often more powerful when used separately. This allows each ingredient to work on the tasks it’s best suited for, rather than neutralizing each other. Understanding their individual strengths will transform your cleaning routine.
| Cleaner | Properties | Common Uses |
|---|---|---|
| Baking Soda | Mild abrasive, deodorizer, cuts grease, pH of 9 (basic) | Scrubbing surfaces, removing odors, cutting through grease, oven cleaning, grout paste |
| Vinegar | Dissolves minerals, removes limescale, cuts through hard water stains, pH of 2-3 (acidic) | Glass cleaning, removing mineral deposits, rust removal, stainless steel shine |
Cleaning with Baking Soda (The Base)
As a mild abrasive, baking soda is excellent for scrubbing surfaces without scratching. Its alkaline nature makes it a natural deodorizer that neutralizes odors, not just masks them. It’s also effective at cutting grease on stovetops and in ovens. For tough jobs, make a paste with water, let it sit, and then scrub. For grout cleaning, a baking soda paste can lift dirt without damaging the grout, making it one of the safest natural cleaners according to UGA’s Green Cleaning guide.
Cleaning with Vinegar (The Acid)
Vinegar’s acidity is perfect for tackling mineral buildup. It easily dissolves minerals that cause white spots on faucets and showerheads. Limescale and hard water stains on glass don’t stand a chance; just apply vinegar, let it sit, and wipe clean. Vinegar can also help with rust removal on small items and is fantastic for restoring a streak-free stainless steel shine on appliances by cutting through fingerprints and water spots.
The Science of the Fizz: What Happens When You Mix Vinegar and Baking Soda?
Remember those childhood volcano experiments that made us feel like mad scientists? That dramatic eruption when vinegar and baking soda meet isn’t just for show – there’s real chemistry happening. Understanding this reaction is the key to figuring out whether this popular cleaning combo actually works or if we’re just watching a fun science experiment in our kitchen sink.
The magic happens because we’re mixing two chemical opposites. Vinegar is an acid (specifically acetic acid), while baking soda is a base (sodium bicarbonate). When they collide, it’s like watching a chemical tug-of-war.
Vinegar packs a punch with its acidity. Most cleaning vinegars contain about 4-10% acetic acid, giving them a pH of around 2-3. That’s pretty acidic! This sourness isn’t just for taste – those acid molecules are excellent at breaking down mineral deposits and cutting through certain types of grime.
Baking soda, on the other hand, sits on the opposite end of the pH scale at around 9. This mild alkalinity makes it fantastic at lifting greasy messes and dissolving stubborn dirt that water alone can’t handle.
When these two meet, the acid from the vinegar grabs onto the bicarbonate from the baking soda, creating carbonic acid. But here’s the thing – carbonic acid is unstable and immediately breaks apart into water and carbon dioxide gas. That’s where all the fizzing comes from!
The reaction is surprisingly powerful. Just one tablespoon of baking soda can produce over five liters of gas when it meets enough acid. It’s quite a reaction that explains why science teachers love this demonstration.
But here’s the million-dollar question: does all that impressive fizzing actually clean better?
The Neutralization Myth: Are You Just Making Salty Water?
Here’s where we need to have an honest conversation about what’s really happening in your cleaning bucket. While that fizzy reaction looks powerful, the end result might surprise you – and not in a good way.
When vinegar and baking soda react, they essentially cancel each other out. The acid neutralizes the base, and the base neutralizes the acid. What you’re left with is mostly water, some carbon dioxide gas (the fizz), and sodium acetate – which is basically a very mild salt.
Think about it this way: you started with a strong acid and a useful base, but now you have… well, slightly salty water. The sodium acetate that forms is actually weaker than the baking soda you started with, so you’ve essentially diluted your cleaning power.
This is what experts like Bill Wuest, PhD explain when they talk about the cleaning effectiveness of this combination. The dramatic fizzing creates a placebo effect – we see all that action and assume it must be cleaning like crazy. But chemically speaking, you’ve just neutralized two perfectly good cleaning agents.
It’s a bit like mixing hot and cold water and expecting it to be more effective than using hot water alone. The chemical properties that made each ingredient useful on its own have been neutralized.
So why do so many people swear by this combination? The answer lies in those bubbles.
The Power of the Bubbles: Mechanical Cleaning Explained
While the chemistry might be disappointing, those bubbles aren’t completely useless. The fizzing action provides what we call mechanical cleaning – think of it as thousands of tiny scrub brushes working away at dirt and grime.
As carbon dioxide gas rapidly forms and escapes, it creates physical agitation that can be genuinely helpful. The bubbles work by loosening debris that’s stuck to surfaces, especially in tight spaces where your scrub brush can’t reach. This makes the combination particularly useful in drains, where the fizzing action can help dislodge hair clogs and soap buildup.
The bubbling also helps with lifting dirt from surfaces. While it won’t tackle heavy-duty stains, it can help separate lighter grime from what it’s stuck to. Think of it like using carbonated water to lift a fresh spill from fabric – the bubbles do some of the work for you.
In confined spaces like drain pipes or narrow bottle necks, this mechanical action becomes more valuable because you can’t easily scrub these areas manually. The bubbles essentially provide the scrubbing action you can’t.
However, let’s be realistic about what this mechanical cleaning can and can’t do. It’s not going to disinfect surfaces, remove serious stains, or handle heavy-duty cleaning tasks. The bubbling action is helpful, but it’s not a miracle worker.
This is why the vinegar and baking soda combination works best for specific tasks like drain maintenance, where the mechanical action is the main benefit you’re after, rather than expecting it to be a general-purpose super-cleaner.
The Power of One: When to Use Them Separately for Best Results
Given that baking soda and vinegar tend to neutralize each other when mixed, our professional advice is often to use them separately. This way, you harness the full power of each ingredient’s unique chemical properties. When used individually, they become incredibly versatile, natural, and effective cleaning agents.
Let’s break down their individual strengths:
| Cleaner | Properties | Common Uses (Source: Multiple).
* Specific Recipe (Refrigerator): “Mix two tablespoons of baking soda with one quart of warm water to clean the inside of refrigerators.” (Source: Blog Post “Why Baking Soda and Vinegar Isn’t the Best Natural Cleaning Solution, According to Chemists”).
* Specific Recipe (Paste): “Make a paste of 2 tbsp baking soda and 1 tbsp water, apply, spray with vinegar, scrub lightly.” (Source: Blog Post “16 things around your house you should be cleaning with baking soda and vinegar”).
* Link: [UGA's Green Cleaning guide](https://extension.uga.edu/content/dam/extension-county-offices/grady-county/4h/doc01548720171113153741.pdf)
Cleaning with Vinegar (The Acid)
- Research Integration: Expand on vinegar’s properties and uses.
- Properties: “mild acid.” “contains around 4–10% acetic acid.” (Source: Multiple).
- Uses: “removing hard-water deposits, discoloration on metal surfaces, and rust stains.” “cleaning laminate floors.” “cleaning shower glass or kettles.” (Source: Multiple).
- Specific Recipe (Faucets): “Soak a paper towel in vinegar and leave it on faucet surfaces with lime deposits to clean them.” (Source: Blog Post “Why Baking Soda and Vinegar Isn’t the Best Natural Cleaning Solution, According to Chemists”).
- Specific Recipe (Laminate Floors): “Mop laminate floors with a mixture of half a cup of white vinegar and a gallon of warm water.” (Source: Blog Post “Why Baking Soda and Vinegar Isn’t the Best Natural Cleaning Solution, According to Chemists”).
- Tone: Practical, helpful.
Effective Cleaning Recipes Using Vinegar and Baking Soda
While not a chemical cure-all, the mechanical action of the vinegar and baking soda fizz is excellent for specific tasks. The key is knowing when to combine them for this effect. For most applications, a ratio of one part baking soda to two parts white vinegar creates a vigorous, dirt-loosening fizz.
Here are three common household tasks where this combination is genuinely effective:

Unclogging Drains with Vinegar and Baking Soda
For minor clogs, the fizzing reaction can help loosen soap scum, hair, and grease, making it a great first line of defense for slow drains.
- Pour hot water down the drain to loosen grease. Caution: Use very hot tap water for PVC pipes, as boiling water can cause damage over time. Boiling water is generally safe for metal pipes.
- Add 1/2 cup of baking soda down the drain, followed immediately by 1 cup of white vinegar.
- Let it sit for at least 10-30 minutes. The expanding gas will agitate the clog.
- Flush with another round of hot water to clear the loosened debris. For greasy kitchen clogs, flush the garbage disposal with cold water to help break up solidified fats.
Deodorizing and Cleaning Toilets & Sinks
The fizzing action and deodorizing power of baking soda are excellent for refreshing toilets and sinks.
- Toilets: Pour 1 cup of baking soda into the bowl, followed by 1 cup of vinegar. Let it fizz and sit for 30 minutes, then scrub and flush to remove odors and minor stains.
- Sinks: Sprinkle baking soda in a damp sink, then spray with vinegar. As it fizzes, use a soft sponge to scrub away grime. For extra shine on stainless steel, place vinegar-soaked paper towels on stains for 20 minutes before rinsing. Find more tips on restoring shine to a stainless steel sink.
- Garbage Disposals: Pour 1/2 cup of baking soda and 1 cup of vinegar down the drain. Let it fizz for 15 minutes, then flush with cold water while running the disposal.
Revitalizing Laundry and Towels
To combat stiff, smelly towels caused by detergent and mineral buildup, use baking soda and vinegar in two separate washes.
- First Wash: Wash towels in hot water with 1 cup of white vinegar (no detergent). This dissolves soap scum and mineral deposits.
- Second Wash: Wash them again in hot water with 1/2 cup of baking soda (no detergent). This will absorb odors and soften the fabric.
This two-step process removes the residue that makes towels feel rough and smell musty. You can also clean your washing machine by running an empty, hot cycle with 1/2 cup of baking soda in the drum and 1 quart of vinegar in the dispenser.
What NOT to Clean & Important Safety Precautions
While vinegar and baking soda are useful, they can damage certain surfaces. Using them incorrectly can lead to costly repairs. Just because they are natural doesn’t mean they are safe for everything.

Surfaces to Avoid with This Duo
The acidic and abrasive properties that make this duo effective can also ruin delicate materials. Avoid using them on:
- Natural Stone: Vinegar’s acid will etch and dull marble, granite, and soapstone. Baking soda can scratch polished stone. Use a pH-neutral cleaner instead.
- Cast Iron: Vinegar will strip away the protective seasoning, leading to rust. Clean with salt and oil.
- Aluminum Cookware: Acid can cause pitting and discoloration.
- Hardwood Floors: Vinegar can damage the protective finish over time, leaving floors cloudy and vulnerable.
- Unsealed Grout: Acid can weaken and discolor unsealed grout. For more on this, see tips for avoiding damage to sealed grout.
- Waxed Surfaces: Vinegar dissolves wax, stripping the protective coating from furniture and floors.
- Rubber Seals & Hoses: The acid in vinegar can degrade rubber components over time, like those in your washing machine.
- Electronic Screens: Never use these cleaners on screens, as they can strip away anti-glare and protective coatings.
Safety First: How to Use Them Safely
Even natural cleaners require caution. Follow these simple safety rules:
- Ventilate: Always work in a well-ventilated area, especially when using vinegar. Open a window or use an exhaust fan.
- Never Mix in a Sealed Container: The buildup of carbon dioxide gas can cause a sealed container to burst. Always mix in an open bowl or sink.
- Wear Gloves: Vinegar can irritate skin, and baking soda can be drying. Protect your hands, especially if you have cuts or sensitive skin.
- Spot Test: Before cleaning a large area, always test a small, inconspicuous spot first to ensure it won’t cause damage.
- Protect Your Eyes: When working with fizzing mixtures, wearing safety glasses is a smart precaution to prevent splashes.
Conclusion: The Verdict on This DIY Cleaning Duo
So, what’s the final word on this popular cleaning combination? While vinegar and baking soda aren’t a miracle solution, they are useful when you understand how they work.
Mixing them creates a neutralizing reaction. The fizz provides a temporary mechanical scrubbing action, but the remaining liquid has little cleaning power. The real strength of these ingredients is often found when they are used separately: baking soda as a gentle abrasive and deodorizer, and vinegar as a powerful acid for dissolving mineral buildup.
However, the combined fizz is perfect for specific tasks like clearing minor drain clogs, deodorizing toilets, or refreshing laundry. Just remember to avoid using them on sensitive surfaces like natural stone, hardwood floors, or cast iron, and never mix them in a sealed container.
At Go Pro Plumbing, we know that sometimes a DIY fix isn’t enough. If you’ve tried this method and are still dealing with a stubborn clog in Sacramento, Rancho Cordova, or anywhere in Northern California, our team is here to help. Our professional drain cleaning services can tackle what home remedies can’t, and we’re ready to provide same-day service when you need us most.
